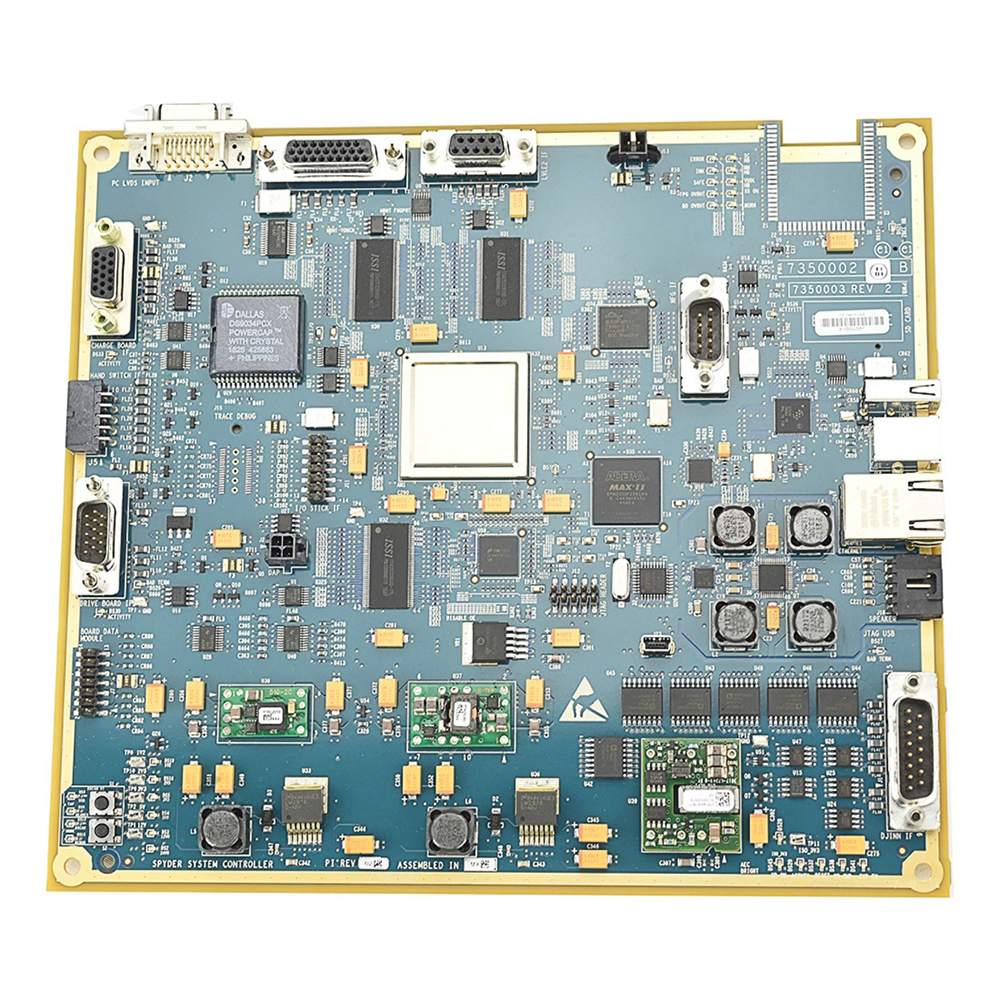
Programmed Spyder System Controller Board 7350002-R
| 7350002-R | |
| Refurbished | |
| X-Ray | |
| GE HealthCare | |
| GE HealthCare | Exchange - Return Defective |
|
Core Exchange | |
Enter your approval number and submit to add item(s) to cart.
Please enter approval number
OR
Don't know your approval number? Call 800-437-1171
Enter opt 1 for the first three prompts, and have your System ID available.
If you add item(s) to cart and submit your order without the
approval number, GE will contact you before your order
can be confirmed for shipment.
Select your approver's name and submit to add item(s) to your cart
Please Select Approver Name
OR
Don't know your approval number? Call 800-437-1171
Enter opt 1 for the first three prompts, and have your System ID available.
If you add item(s) to cart and submit your order without
selecting an approver, GE will contact you before your order
can be confirmed for shipment.
Features
- Hazardous materials are not used
- Equipped with electronic circuit breaker for overcurrent protection
- Low melting point board material
- Meets international standards for safety requirements
Product Overview
The Programmed Spyder System Controller Board is a custom designed circuit board, intended for use in XR Optima modules and other medical equipment as applicable. The control board assembly is comprised of various components including buffers, connectors, capacitor, fuse, logic interface, semiconductor diode, IC's, switch, resistor, surface mount pin-strip header, LED, quartz crystal, oscillator, ferrite bead, regulator, logic gate, transistor, memory SDRAM, ethernet PHY transceiver, USB and battery. The assembly is used as a Field Replacement Unit (FRU) for over hauling or servicing. The board is made of a high quality polycaprolactone material which is a biodegradable polyester that has a low melting point. The Spyder assembly complies to regular functional checks and insulation tests. The circuit board is compatible to operate with XR Optima XR200amx, XR Optima XR220amx, XR Brivo XR285amx, XR Mobile DR and other modules as applicable. The assembly is a proven design which fits well into many demanding applications. The product's high quality material helps to offer reliable operation, efficient functioning and longer life span.
Return And Exchange
GE HealthCare will accept, for return from customer, items that are in new condition, unworn, unaltered and free of damage. Items cannot be returned or exchanged beyond 30 days from customer’s receipt of the original order and all returned items will be subject to a 15% restocking fee. To obtain a full refund or exchange of the item within the 30 day return period (subject to the 15% restocking fee) customer must request a return materials authorization (RMA) from GE HealthCare and return the item to GE HealthCare utilizing this RMA number. Items must be returned in the original packaging, unused and free of damage.
Please note, cuffs, BP items, sterile and hazmat items cannot be returned. If a replacement order is requested, the restocking fee may be waived at GE HealthCare’s sole discretion.