Bite Hole Indicator KZ200800
| KZ200800 | |
| Neuf | |
| Ultrason | |
| Other Ultrasound | |
| GE HealthCare | |
| GE HealthCare | |
| N/A | Outright |
Enter your approval number and submit to add item(s) to cart.
Please enter approval number
OR
Don't know your approval number? Call 800-437-1171
Enter opt 1 for the first three prompts, and have your System ID available.
If you add item(s) to cart and submit your order without the
approval number, GE will contact you before your order
can be confirmed for shipment.
Select your approver's name and submit to add item(s) to your cart
Please Select Approver Name
OR
Don't know your approval number? Call 800-437-1171
Enter opt 1 for the first three prompts, and have your System ID available.
If you add item(s) to cart and submit your order without
selecting an approver, GE will contact you before your order
can be confirmed for shipment.
Features
- Good dimensional stability
- High corrosion resistance
- High accuracy
- High strength and stiffness properties
Fréquemment acheté avec cet article
Présentation du produit
The Bite Hole Indicator is a device designed for detection of bite holes in the TEE probe endoscope. The bite hole indicator comes with three leads (red x 2, black x 1) and a copper plate with cable. The bite hole Indicator uses a 9V PP3 battery situated in a compartment on the rear of the device. The probe endoscope which is to be tested, is immersed in a saline solution (50g NaCl per 1 liter water) inside a water bath. The red leads of the indicator are connected to the metal parts of the endoscope and the black leads is connected to the copper plate which is immersed in the saline solution. The text and graphics on the indicator are scaled precisely to size.
Produits compatibles
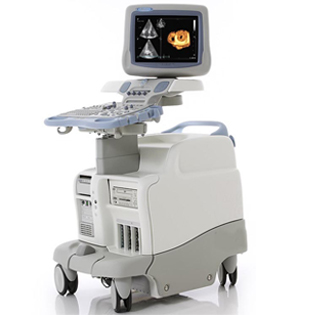
Vivid 7
The Vivid 7/Vivid 7 PRO ultrasound unit is a high performance digital ultrasound imaging system.The system provides image generation in 2D (B) Mode, Color Doppler, Power Doppler (Angio), M-Mode, Color M-Mode, PW and CW Doppler spectra, Tissue Velocity imaging and Contrast applications. The fully digital architecture of the Vivid 7/Vivid 7 PRO unit allows optimal usage of all scanning modes and probe types,throughout the full spectrum of operating frequencies.The Vivid 7 Dimension gives clinicians a better way to convey their findings to other cardiologists, referring physicians,EP physicians and patients. Now, cardiac anatomy,synchronicity and viability can be clearly communicated in imaging formats that are more familiar for your clinical partners, thus easier to understand.
• Real-time 4D imaging – provides more cardiac information to help clinicians better communicate the heart’s structure and function.
• 4D Tissue Synchronization Imaging (TSI) – propels Tissue Velocity Imaging (TVI) to the next level by taking three simultaneous planes – from a single heartbeat at high frame rates – to create a flexible, dynamic 4D model with quantitative measurements to better communicate cardiac dyssynchrony.
• Bull’s-eye report formats and TSI surface mapping –communicate cardiac dyssynchrony in a visual display that should be more familiar to EP physicians.
• Blood Flow Imaging (BFI) – new vascular imaging mode gives clinicians a better understanding and delineation of directional blood flow in vessels.
• Seamless measurement integration – allows you to efficiently calculate ejection fraction and volumes from tri-plane images gathered from the same heartbeat.
Return And Exchange
GE HealthCare will accept, for return from customer, items that are in new condition, unworn, unaltered and free of damage. Items cannot be returned or exchanged beyond 30 days from customer’s receipt of the original order and all returned items will be subject to a 15% restocking fee. To obtain a full refund or exchange of the item within the 30 day return period (subject to the 15% restocking fee) customer must request a return materials authorization (RMA) from GE HealthCare and return the item to GE HealthCare utilizing this RMA number. Items must be returned in the original packaging, unused and free of damage.
Please note, cuffs, BP items, sterile and hazmat items cannot be returned. If a replacement order is requested, the restocking fee may be waived at GE HealthCare’s sole discretion.